You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Harsh hop bitterness
- Thread starter welly2
- Start date

Help Support Australia & New Zealand Homebrewing Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
manticle
Standing up for the Aussie Bottler
Have you used those hops in a similar way before?
Is it astringency you are getting? I was getting astringency in pretty much all of my brews. I was screwing around with water too much and since backing right off on the water side of things, my brews have improved immensely. There was a batch of IIPA I brewed recently, which was very astringent post dry hopping, that I tipped. I dry hopped it after it had reached FG and after it was taken off the yeast. My current thoughts are that because the yeast had already finished it's job, the harsh astringency of raw hops (try eat some one day, you'll know what I mean) was carried over into the beer. My thinking is that the magic of yeast , whilst active, does wonderful things to astringency, and that dry hopping should occur just prior to FG to let that magic take place. I do want to have a chat with some pro brewers to see if my theory is correct though - and see how they do it.
Top idea,Danscraftbeer said:If cold crashing my opinion is that it should be air tight so it doesn't draw in any air.
That also means the walls of a plastic fermenter will cave in when it contracts.
Added 2c I made Stainless Steal plugs that fit tight in the airlock grommet. 30mm long 10mm thick stainless round rod rounded off one end and polished. Oh the things we do....
I misplaced one of my thermowells and plugged the second grommet with a 12mm barb with a stainless cap on the end. Not as elegant due to all the angles and threads.
I wish I had a lathe. Men have lathes.
TheWiggman
Haters' gonna hate
Actually fair point by manticle, welly commented that he's having bitterness issues with his beers. Maybe this is what we should be discussing rather than a highly questionable web post?
What's your process welly2, are there specific beers you are getting the issue with?
What's your process welly2, are there specific beers you are getting the issue with?
zorsoc_cosdog said:There have been a couple of pointers to the water. How could this influence bitterness?
Chloride / Sulfate ratio.
Too much sulfate may enhances bitterness.
I was using RO/DI water, then adding various combinations of salts to end up with a predetermined water profile. The salts I was using were some, or all of the following: Calcium Chloride and Sulphate, Magnesium Sulphate, Chalk and Bicarbonate of Soda. When changing to RO (not RO/DI) water, mixed with various percentages of Perth tap water, my astringency (as opposed to bitterness) issues largely disappeared. Why? I don't know for sure; however the positive change has been noted by myself, and others.zorsoc_cosdog said:There have been a couple of pointers to the water. How could this influence bitterness?
rude
Well-Known Member
- Joined
- 7/5/08
- Messages
- 1,442
- Reaction score
- 216
I was using Melville water high in sodium ,chloride I backed right off on the hops
because of the bitterness harshness
A English bitter I was going from 32 IBU to 25
Since R,O water use beers have been coming out malty can actually taste the yeasts flavours,
have had to readjust hop schedule where Im thinking maybe a touch more than 32 IBUs
It may have been astringency from high PH ? but it has made a big difference
Also not as dry after a session
Before I'de wake up dry as a Nuns nasty
Note I'm a no chiller & have been just hopping in the kettle lately but have cubed hopped before
because of the bitterness harshness
A English bitter I was going from 32 IBU to 25
Since R,O water use beers have been coming out malty can actually taste the yeasts flavours,
have had to readjust hop schedule where Im thinking maybe a touch more than 32 IBUs
It may have been astringency from high PH ? but it has made a big difference
Also not as dry after a session
Before I'de wake up dry as a Nuns nasty
Note I'm a no chiller & have been just hopping in the kettle lately but have cubed hopped before
Danscraftbeer
Well-Known Member
How one questionable harsh flavour turns into a can of worms. :lol:
Notes, notes, take notes etc, more notes, and take more notes and judge of your own beers.
My beers are more malty these days but with a bitter enough backbone to them as well. On tap 20 days from grain to brain.
I need more kegs for longer conditioning.
Notes, notes, take notes etc, more notes, and take more notes and judge of your own beers.
My beers are more malty these days but with a bitter enough backbone to them as well. On tap 20 days from grain to brain.
I need more kegs for longer conditioning.
manticle
Standing up for the Aussie Bottler
Soon I hope to buy a lathe for my workplace so I can make sexy things from aluminium and stainless.zorsoc_cosdog said:I wish I had a lathe. Men have lathes.
quadbox
Well-Known Member
- Joined
- 30/9/07
- Messages
- 221
- Reaction score
- 39
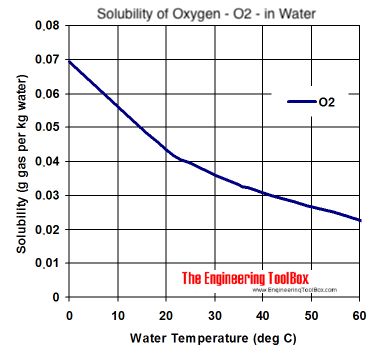
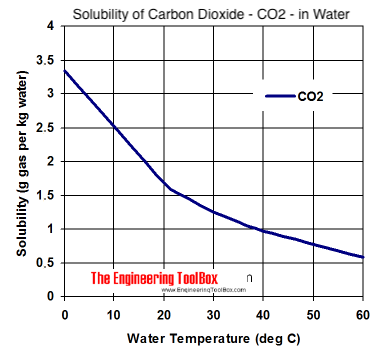
I was looking for molar solubility graphs, but close enough for internet handwaving points regardless. The solubility of oxygen at 0C (crash chilling temps) is still utterly miserable compared to co2. Will 0.07grams of oxygen per kilogram of beer oxidise more than 0.04 grams? Hell yes. Will that be measurable compared to other factors involved at the early stages of conditioning? Lol.
The entire "CO2 blanket protects your beer" thing has been debunked very thoroughly, very often, but wow does it forever rise from the grave.
manticle
Standing up for the Aussie Bottler
I sleep with a co2 blanket.
Danscraftbeer
Well-Known Member
That graph reassures my suspicions. I'll advocate for no exposures of your beer is best. I'll go the hop stands instead of throwing dry pellets or flowers into a post ferment beer.
Matplat
Well-Known Member
- Joined
- 15/1/15
- Messages
- 1,233
- Reaction score
- 451
Please elaborate.... I haven't heard of this debunking previously?
Seems plausible enough to me that in the same way salad dressing separates in the jar, an undisturbed atmosphere in the top of your FV will do the same thing with CO2 and any remaining air?
Isn't that what allows open top fermentation vessels to work?
Seems plausible enough to me that in the same way salad dressing separates in the jar, an undisturbed atmosphere in the top of your FV will do the same thing with CO2 and any remaining air?
Isn't that what allows open top fermentation vessels to work?
manticle
Standing up for the Aussie Bottler
My understanding is that co2 offers protection while it's being produced during fermentation (some - it's not a mylar or cryovac barrier). However once fermentation slows or stops, your blanket gets thinner every second.
Some brewers seem to think they could have house guests sleep under it for half a year.
Some brewers seem to think they could have house guests sleep under it for half a year.
Danscraftbeer
Well-Known Member
Yeah. Then when your beer cools it absorbs that Co2 layer. Then it probably absorbs your used fridge air if its not air tight.
Edit: my notes; A standard 30lt plastic fermenter with a 20lt brew. Cold chilling will draw in a basket ball worth of volume of air.
Cant rely on a Co2 layer there.
Edit: my notes; A standard 30lt plastic fermenter with a 20lt brew. Cold chilling will draw in a basket ball worth of volume of air.
Cant rely on a Co2 layer there.
GalBrew
Well-Known Member
You need to keep in mind gas partial pressures when the magical co2 blanket is discussed.
I thought about this but realised that the sun's energy leads to atmospheric turbulence and therefore mixing of the gases.mckenry said:Put simply if CO2 and air didn't mix, we'd all be dead. The 'protective layer' would be on earth and the air would be above that.
Not the same for the fermenter. There must be a property of dissimilar gases to homogenise. Is it just entropy? Is the rate of diffusion less than the natural mixing due to the high temperature (yes, I mean 0C) of the gases?
Similar threads
- Replies
- 0
- Views
- 2K
- Replies
- 12
- Views
- 4K



