Goose
0 Warning Points
- Joined
- 6/7/05
- Messages
- 638
- Reaction score
- 147
Gents, I don't mean this to be yet another diacetly thread, but having repeated issues with what appears to be a delayed formation of diacetyl in the last two lagers I have done.
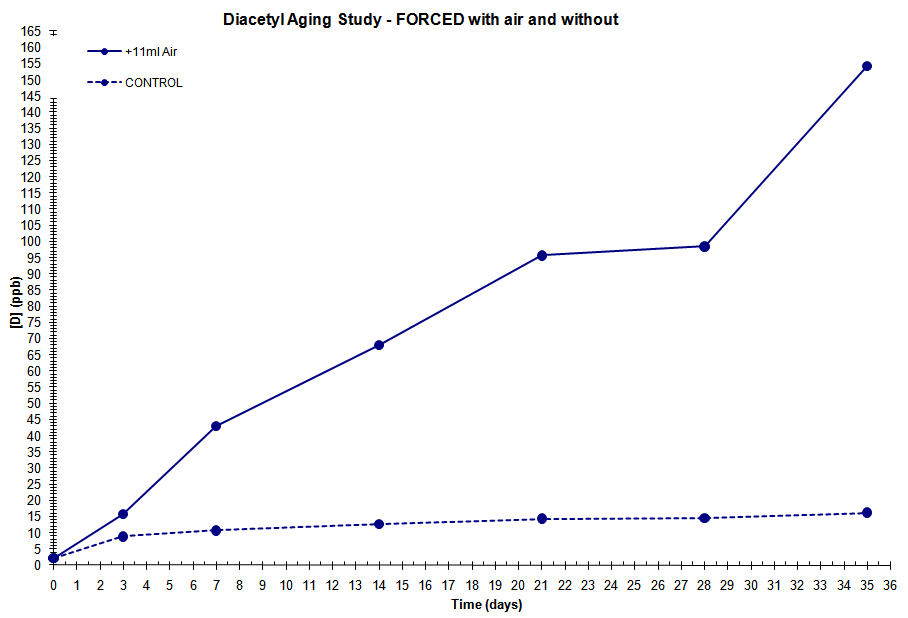
What happens is that there appears to be no trace of diacetly right up to the racking stage, and it appears only after I have carbonated up the beer. My fermentation process has been 3 weeks at a steady 10 deg C using a fresh Wyeast 2042 Danish Lager made up to a 4 litre starter. Beer tastes fantastic, clean, clear and fresh prior to racking and I can detect no diacetly at all. Then, I rack, chill to 2 deg C and slow force carbonate over 10 days at 12 psi. I regularly taste and can detect no off taste, but afer a few days I can detect a hint , and it gradually gets stronger with time. After full carbonation, its positively gross. So now, no choice but to try and get it reabsorbed by raising the temperature to 18 deg c for a few days. if that fails I fear it will become fertiliser.
At first I thought the diacetyl was being created from residual yeast that get affected by the force carbonation pressure which I usually do, ie 2 days at around 25 psi. Seems even the low pressure carbonation has the same end result.
So a tad puzzled by this, I'd always assumed a diacetyly rest was necessary only if diacetyl was present and detectable, and raising the temperature would cause the yeast to consume and metabolise it. However its creation post racking / chilling / carbonation is really p*ssing me off...
any experienced lager brewers out there that can shed some light ?
What happens is that there appears to be no trace of diacetly right up to the racking stage, and it appears only after I have carbonated up the beer. My fermentation process has been 3 weeks at a steady 10 deg C using a fresh Wyeast 2042 Danish Lager made up to a 4 litre starter. Beer tastes fantastic, clean, clear and fresh prior to racking and I can detect no diacetly at all. Then, I rack, chill to 2 deg C and slow force carbonate over 10 days at 12 psi. I regularly taste and can detect no off taste, but afer a few days I can detect a hint , and it gradually gets stronger with time. After full carbonation, its positively gross. So now, no choice but to try and get it reabsorbed by raising the temperature to 18 deg c for a few days. if that fails I fear it will become fertiliser.
At first I thought the diacetyl was being created from residual yeast that get affected by the force carbonation pressure which I usually do, ie 2 days at around 25 psi. Seems even the low pressure carbonation has the same end result.
So a tad puzzled by this, I'd always assumed a diacetyly rest was necessary only if diacetyl was present and detectable, and raising the temperature would cause the yeast to consume and metabolise it. However its creation post racking / chilling / carbonation is really p*ssing me off...
any experienced lager brewers out there that can shed some light ?