Storing yeast on malt-agar slants is easy enough to do, and it works well, however it takes time and effort, especially since the yeast should be re-slanted every 6 months.
Since last year, I've been freezing yeast samples with the aim of reducing the work required to archive yeast strains and keep them for long periods. If done right, the frozen yeast should be able to be stored for at least 2 years and maybe even 5+ years.
The method I have been using pre-dates the publication of the 'Yeast' book, however, it is very similar to what they suggest and I did get a few pointers from JZ.
Other than using malt-agar slopes and plastic test-tubes and vials there is no need for special lab-equipment, but a centrifuge and YPD solution are recommended in the book.
The idea behind freezing yeast is that the lower the temperature the longer the yeast should keep without mutation and changes. Cryo-storing at the yeast -80degC would be ideal since at that temperature the yeast should (if frozen successfully) keep for an indefinite period, however like most people all I have is a normal (but non-frost-free) freezer that sits at about -18 to -20degC.
Unlike BribieG's first attempt to freeze yeast, the method I use is not focused on storing pitch-able quantities of yeast, so it's a little more time-consuming since the yeast is slanted (to confirm and check viability) both before and then after it's been frozen and then a starter is made from the slant prepared from the previously frozen sample.
The viability and vitality of the yeast before it is frozen plays a factor with how well it can be stored, so I always start with a freshly inoculated slant of yeast:

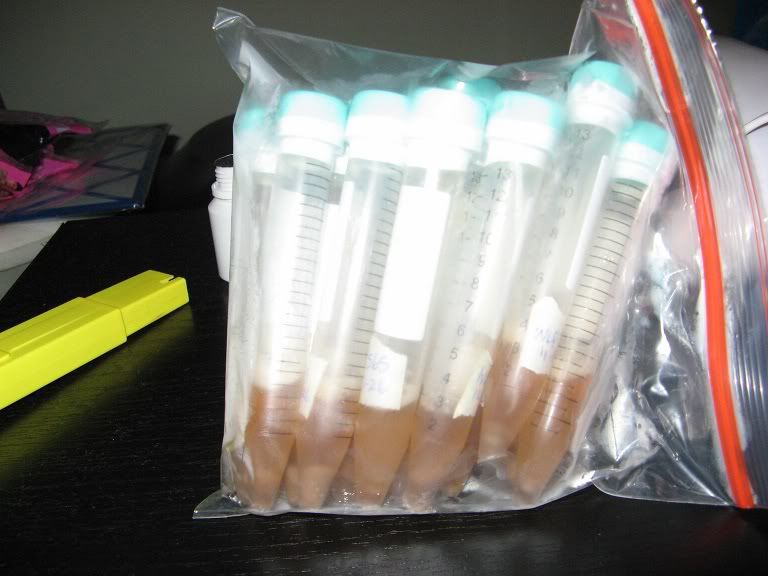
10ml of sterile wort (about 1.035 gravity) is prepared in 15ml plastic test-tubes.
I make the wort and fill the tubes in batches before putting them in the pressure cooker to 'autoclave', once that is done they should then be sterile and keep indefinitely.

The sterile wort is tipped into the slanted-vial and the yeast scraped off the malt-agar with an inoculation loop.

Tubes are capped and then shaken to aerate:

Over the next 3-5 days the yeast should fully ferment out in the 10ml of wort.
Test tube caps are tightened to shake the samples frequently, but then loosened so that pressure does not build up that might harm the yeast.

.... ctd next post
Since last year, I've been freezing yeast samples with the aim of reducing the work required to archive yeast strains and keep them for long periods. If done right, the frozen yeast should be able to be stored for at least 2 years and maybe even 5+ years.
The method I have been using pre-dates the publication of the 'Yeast' book, however, it is very similar to what they suggest and I did get a few pointers from JZ.
Other than using malt-agar slopes and plastic test-tubes and vials there is no need for special lab-equipment, but a centrifuge and YPD solution are recommended in the book.
The idea behind freezing yeast is that the lower the temperature the longer the yeast should keep without mutation and changes. Cryo-storing at the yeast -80degC would be ideal since at that temperature the yeast should (if frozen successfully) keep for an indefinite period, however like most people all I have is a normal (but non-frost-free) freezer that sits at about -18 to -20degC.
Unlike BribieG's first attempt to freeze yeast, the method I use is not focused on storing pitch-able quantities of yeast, so it's a little more time-consuming since the yeast is slanted (to confirm and check viability) both before and then after it's been frozen and then a starter is made from the slant prepared from the previously frozen sample.
The viability and vitality of the yeast before it is frozen plays a factor with how well it can be stored, so I always start with a freshly inoculated slant of yeast:

10ml of sterile wort (about 1.035 gravity) is prepared in 15ml plastic test-tubes.
I make the wort and fill the tubes in batches before putting them in the pressure cooker to 'autoclave', once that is done they should then be sterile and keep indefinitely.

The sterile wort is tipped into the slanted-vial and the yeast scraped off the malt-agar with an inoculation loop.

Tubes are capped and then shaken to aerate:

Over the next 3-5 days the yeast should fully ferment out in the 10ml of wort.
Test tube caps are tightened to shake the samples frequently, but then loosened so that pressure does not build up that might harm the yeast.

.... ctd next post